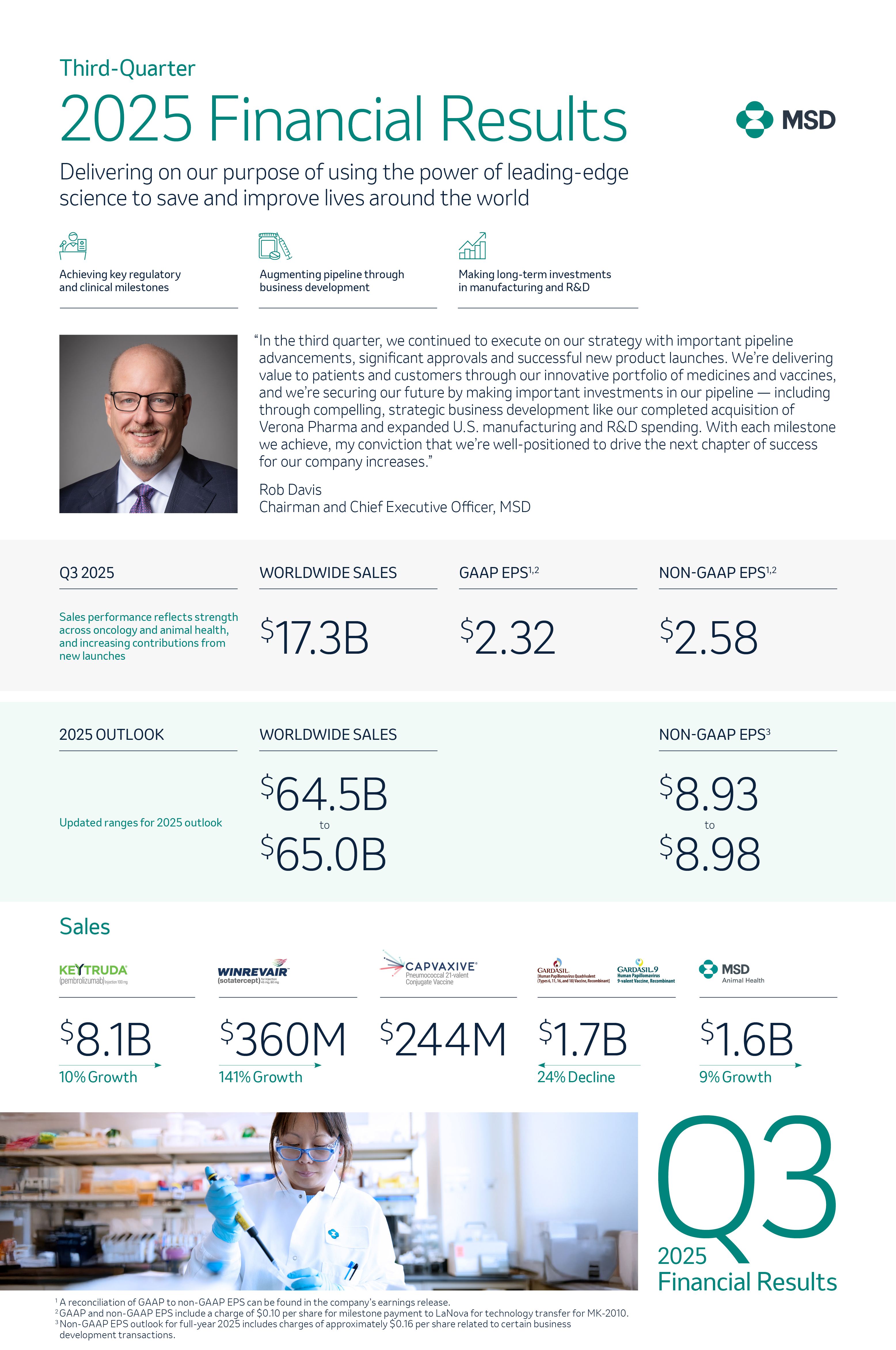
5 ways we’re transforming artificial intelligence into impact
We’re applying AI across our company to help us work smarter and faster so we can reach patients sooner

At MSD, we’re in the business of knowledge, insights and innovation — rooted in intelligence.
Today, artificial intelligence (AI) — or what could also be “automated”, “accelerated” or “augmented” intelligence — lies not only in software and computer systems, but in the data, development and delivery of these intelligent tools to achieve better outcomes for patients.
Here are five ways we’re using AI to drive our purpose of saving and improving lives around the world.
01.
Accelerating the discovery of new medicines
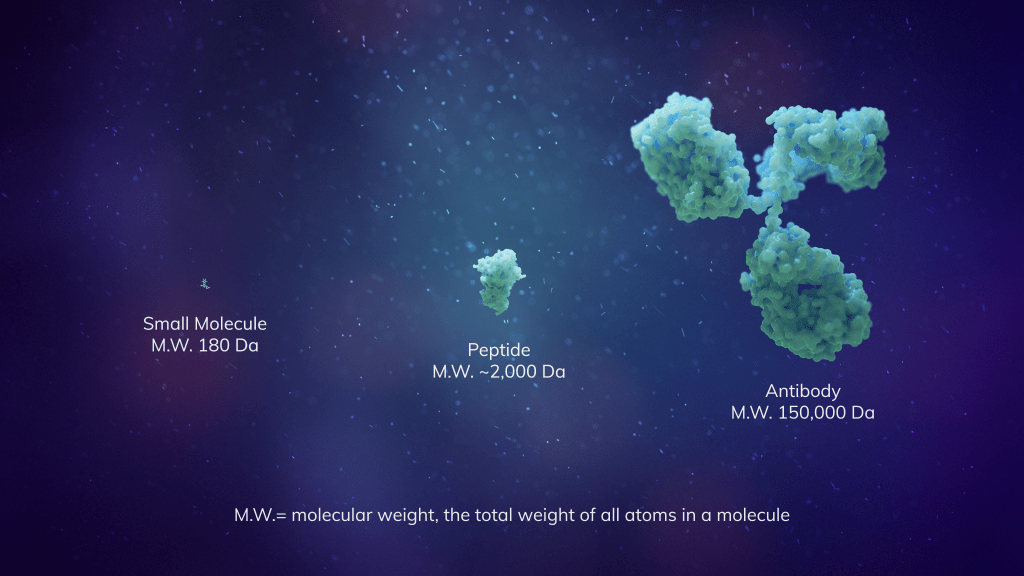
Drug discovery remains an endeavor where only about 1 in 10 drug candidates that enter clinical trials ultimately receive regulatory approval. We’re working to change that by enabling scientists to use AI and machine learning (ML) foundation models to enhance and build upon their existing approaches to drug design before experimental testing and clinical trials.
We recently developed two foundation models which uncover patterns in disease to find better drug targets, allow faster molecular design and rapidly test small molecules, including cyclic peptides, for efficacy and toxicity before going into the clinic.
By unlocking patterns within vast datasets, these AI models enable our scientists to accelerate the discovery of leading therapeutic candidates — a process that normally takes 10 years — allowing us to potentially get therapies to patients faster without compromising scientific rigor.
02.
Optimizing clinical trials
Enrolling people in clinical trials and keeping them engaged once they’ve signed up remains a significant challenge across our industry, with approximately 20% of activated sites failing to enroll a single participant. We’re addressing this by using AI to help improve site selection, patient matching and retention. For example, predictive models can flag patients at higher risk of dropping out, enabling targeted interventions that improve retention and keep trials on track.

03.
Automating workflows to improve productivity
Our enterprise-wide training program helps employees understand the latest digital technology, including generative and agentic AI, and learn how to use it responsibly. Our proprietary AI platform — which more than 80% of our workforce uses — applies large language models to enable employees to automate, simplify and digitize processes that historically took more time, freeing us up to prioritize more impactful work.

04.
Modernizing manufacturing
Generative AI helps protect our supply chain when potentially disruptive events like natural disasters or port delays occur. Our systems can produce event-based risk assessments in under 30 minutes — allowing us to quickly see which products and sites are affected and act to avoid or reduce shortages and delays.
In vaccine manufacturing, we’re using computer vision — another form of AI — to inspect vials and syringes for defects. This results in less waste, lower costs and higher production speed.
05.
Streamlining education and engagement with health care providers
We’re using AI to streamline information for providers and patients to ensure we deliver the right details to the right people when it matters most.
We’ve embedded AI across the content life cycle — from conception through medical, legal and regulatory review — so that we can organize messages more intelligently. The result: higher quality, personalized content that gets to health care providers faster.
Supporting this is our generative AI-powered chatbot for our field representatives. It summarizes relevant insights and helps us respond in real time to provider needs.

It all starts with data
Data powers AI. We have a vast repository of proprietary and secure data, but for it to be usable, it must first be structured and organized.
We’re continuously working to create a frictionless data flow so AI can reliably and accurately drive faster, more targeted and personalized outcomes.
Data is critical to our business strategy and to our pipeline. When our data is high-quality, well-manicured and organized to support powerful insights, we can make more accurate and intelligent predictions — and move faster to deliver the medicines and vaccines patients are waiting for.
Read more about how we’re using data science, AI and machine learning.