Vaccine inventors, creators and innovators
Dr. Maurice Hilleman was among pioneering scientists who made strides in vaccine history and the fight against infectious disease
May 8, 2024
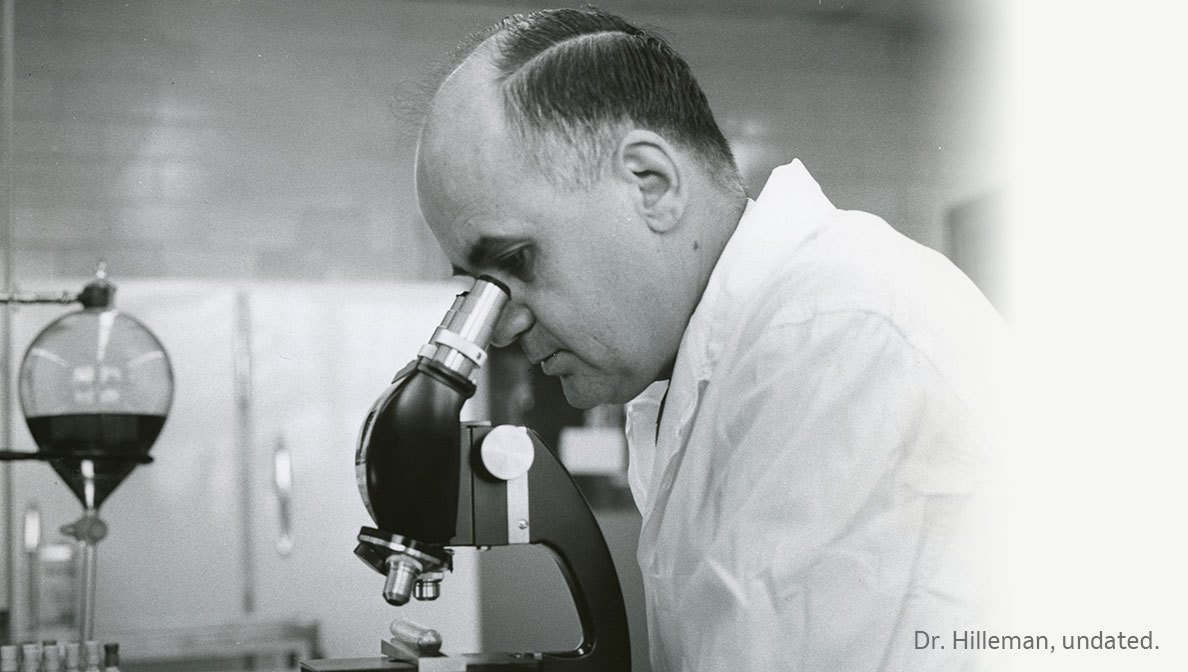
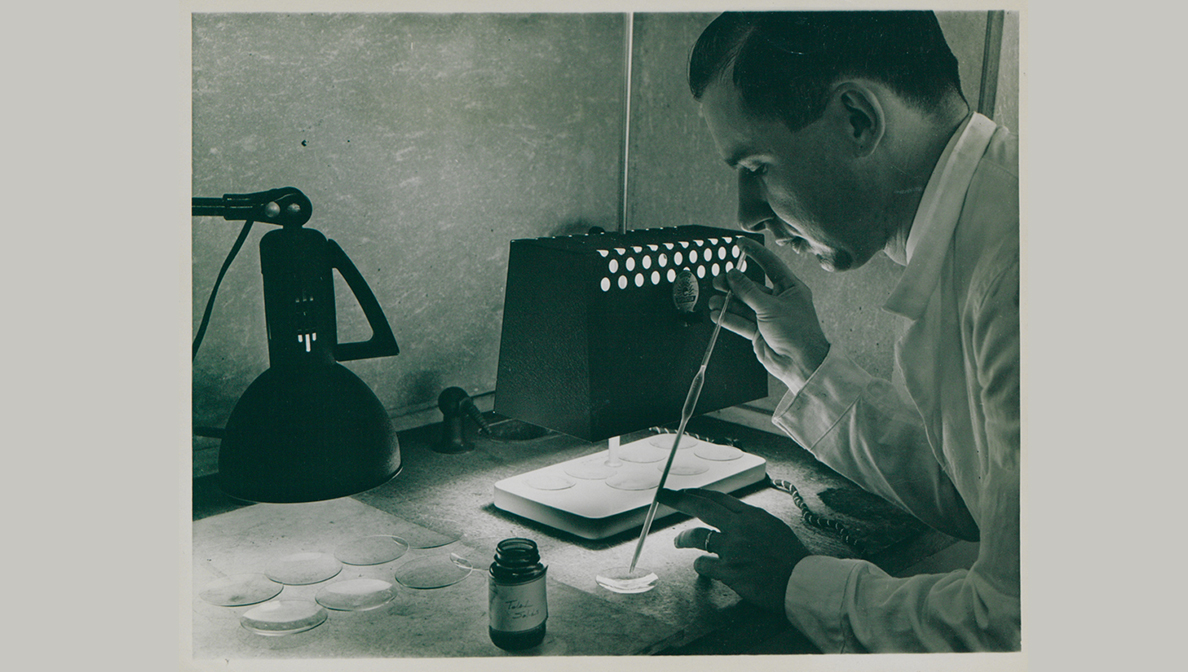
Dr. Maurice Hilleman, who led our department of virus and cell biology from 1957 to 1984.
When were vaccines invented?
The story of modern day vaccines began in 1796 when Dr. Edward Jenner inoculated 9-year-old James Phipps with cowpox as a way to protect him from smallpox. The term ‘vaccine’ is later coined, taken from the Latin word for cow, vacca. Smallpox was the first disease people tried to prevent by intentionally inoculating themselves with infected matter.

Dr. Edward Jenner inoculating 9-year-old James Phipps with cowpox.
Eight decades after Jenner published his findings, Louis Pasteur developed the first live attenuated rabies vaccine. Attenuation is a process that weakens the bacteria or virus in a vaccine so it’s less likely to cause disease, while still triggering an immune response similar to the natural infection. During the mid- to late-20th century, advances in basic and clinical research made it possible for scientists to develop vaccines to help protect against both bacterial and viral diseases.
Dr. Maurice Hilleman’s contribution to vaccine development
The names Jonas Salk and Albert Sabin have become synonymous with their inventions and developments around the polio vaccine, and the giant strides they made in the fight against viral diseases. Although these are some of the most famous names in vaccine research, MSD has a legacy of vaccine pioneers, too. Dr. Maurice Hilleman, who led MSD’s department of virus and cell biology from 1957 to 1984, also belonged to that distinguished group. Credited with helping to develop more than 40 experimental and licensed human and animal vaccines, Hilleman’s passionate commitment continues to inspire scientists in medical research laboratories to this day.
Hilleman was born and raised on a farm in Montana. It was a hard life, but a farm background was a great foundation for his later work.
“When you’re brought up on a farm, you have a lot of general knowledge,” he said. After graduating from the University of Chicago with a doctorate in microbiology and chemistry, Hilleman chose to work at a pharmaceutical company instead of academia.
Despite his many accomplishments, including helping to develop more than 40 human and animal vaccines, Hilleman’s name is virtually unknown by the general public and press. Yet his impact on public health is undeniable.
“His commitment was to make something useful and convert it to clinical use. Maurice’s genius was in developing vaccines, reliably reproducing them, and he was in charge of all pharmaceutical facets from research to the marketplace.”
- Dr. Paul Offit
Director of the Vaccine Education Center, professor of pediatrics in the Division of Infectious Diseases at Children’s Hospital of Philadelphia, and Hilleman’s biographer
In 1988, President Ronald Reagan awarded Hilleman the National Medal of Science, and in 1997, he was honored with the Albert B. Sabin Gold Medal Award. Dr. Anthony Fauci, former director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, called Hilleman “one of the true giants of science, medicine and public health in the 20th century.”